News
So-called junk DNA plays critical role in mammalian development (Lin He Lab)
By Robert Sanders | October 18th, 2021
Nearly half of our DNA has been written off as junk, the discards of evolution: sidelined or broken genes, viruses that got stuck in our genome and were dismembered or silenced, none of it relevant to the human organism or human evolution.
But research over the last decade has shown that some of this genetic “dark matter” does have a function, primarily in regulating the expression of host genes — a mere 2% of our total genome — that code for proteins. Biologists continue to debate, however, whether these regulatory sequences of DNA play essential or detrimental roles in the body or are merely incidental, an accident that the organism can live without.
A new study led by researchers at University of California, Berkeley, and Washington University explored the function of one component of this junk DNA, transposons, which are selfish DNA sequences able to invade their host genome. The study shows that at least one family of transposons — ancient viruses that have invaded our genome by the millions — plays a critical role in viability in the mouse, and perhaps in all mammals.
When the researchers knocked out a specific transposon in mice, half their mouse pups died before birth.
This is the first example of a piece of “junk DNA” being critical to survival in mammals.
In mice, this transposon regulates the proliferation of cells in the early fertilized embryo and the timing of implantation in the mother’s uterus. The researchers looked in seven other mammalian species, including humans, and also found virus-derived regulatory elements linked to cell proliferation and timing of embryo implantation, suggesting that ancient viral DNA has been domesticated independently to play a crucial role in early embryonic development in all mammals.
According to senior author Lin He, UC Berkeley professor of molecular and cell biology, the findings highlight an oft-ignored driver of evolution: viruses that integrate into our genome and get repurposed as regulators of host genes, opening up evolutionary options not available before.
Read the full article at UC Berkeley News.
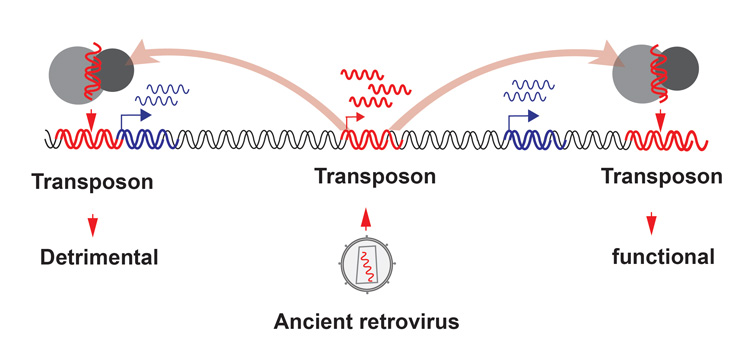
Image description: Viruses have been invading the genomes of animals and plants since time immemorial (center), but scientists have assumed that most of them were quickly silenced, building up a graveyard of transposons that today make up the bulk of our DNA. Researchers are finding, however, that some transposons have copied themselves into spots in the genome that have harmful effects (left). Conversely, other transposons have taken on a role as regulators of important functions, such as development (right). (Graphic courtesy of Lin He lab)
Tumors Disrupt the Blood-Brain Barrier at a Distance (David Bilder Lab)
By Abby Olena | September 9th, 2021The goal of most cancer therapeutics is to eliminate or at least stop the growth of malignant cells. A study published September 7 in Developmental Cell points to a possible complementary strategy: reinforcing host defenses, specifically the blood-brain barrier (BBB). The authors found that tumors in the body can disturb the BBB and that interfering with this disruption seems to improve host health in both fruit flies and mice, even as tumor growth continues.
This work “shows that [breaking] the blood-brain barrier is at least one of the causes of death” in animals with cancer, says Wu-Min Deng, who studies tumorigenesis in Drosophila at Tulane University School of Medicine and was not involved in the study. It’s “a very good example of how the fly model can be used to answer some very difficult questions in cancer biology,” he adds.
For the past two decades or so, David Bilder’s group at the University of California, Berkeley, has leveraged Drosophila models to better understand cancer. Then, “maybe eight or nine years ago, a really spectacular student came to the lab, who got very interested in the idea of that we could use the fly not only as a model for studying how tumors grow, but also how tumors affect the host,” he tells The Scientist.
In 2015, that student, Alejandra Figueroa-Clarevega, and Bilder showed in fruit flies that malignant tumors make a protein that disrupts insulin signaling, leading to so-called cachexia, a breakdown of fat and muscle and subsequent extreme weight loss, which is linked to death in many human cancer patients. The work inspired current Bilder lab postdoc Jung Kim, who led the new study, to use the fly to explore other ways in which a tumor affects a host at distant sites in the body.
Kim, Bilder, and colleagues started by transplanting tissue that expresses the activated oncogenes Ras and aPKC into wildtype adult flies, where the tissue formed malignant, nonmetastatic tumors. These tumors killed the animals within a few weeks—about half the lifespan of flies that received a noncancerous tissue transplant. The researchers determined that these tumors activate the JAK/STAT signaling pathway—a readout of inflammation—throughout the flies and particularly in glial cells.
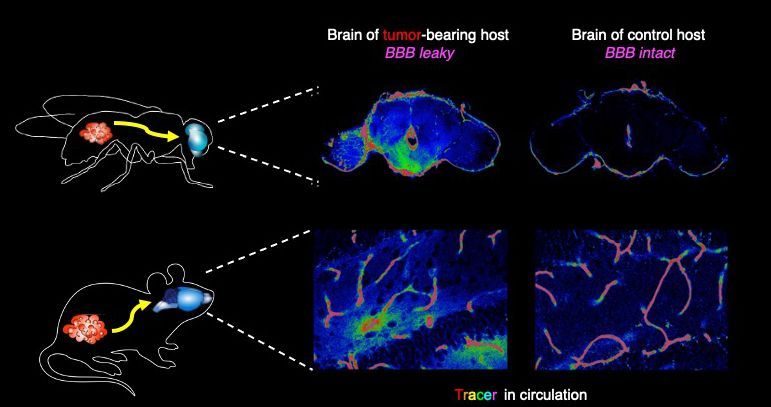
The BBB in flies is made of glia that protect the brain from the open circulation of hemolymph, the circulatory fluid in Drosophila. The researchers checked whether the BBB was intact by injecting a dye into the hemolymph. They found that the dye got into the brains of flies with tumors but not the brains of controls.
Next, the team knocked out the fly orthologs of interleukin 6 (IL-6), a cytokine that can activate JAK/STAT signaling; the receptor that the IL-6 orthologs bind to; or STAT itself in graft or host tissue. When they did that, the breakdown of the blood brain barrier didn’t happen in the flies with cancer, and the insects lived longer. Activation of JAK/STAT signaling in the glia in the absence of a tumor also led to an increase in BBB permeability, followed by eventual death, though it happened much more slowly than in flies with tumors. All together, the authors conclude, these findings indicate that cytokines produced by the tumor lead to inflammation and disruption of the BBB, which ultimately kills the host.
The effects of preserving the BBB in flies are “really remarkable. They’re living longer in the presence of a tumor,” Bilder tells The Scientist.
Read the full article at The Scientist Magazine.
Image description: In flies and mice, cytokines released from tumors in the body cause cell junctions that normally protect the brain from circulating molecules to open up, shown here by the diffusion of a tracer dye. Image by Jung Kim, Hsiu-Chun Chuang, David Bilder.
Martik honored with AHA Career Development Award
July 7th, 2021
Assistant Professor of Genetics, Genomics and Development Megan Martik has been awarded a Career Development Award from the American Heart Association (AHA) for her proposal, "Differentiation of the cardiac neural crest into specialized derivatives." The award supports highly promising healthcare and academic early-career professionals exploring innovative questions or pilot studies that will contribute to their future success as research scientists.
Martik will investigate the genetic interactions driving the formation of the cardiac neural crest and subsequent differentiation into cell types such as cardiomyocytes by uncovering the underlying gene regulatory networks (GRNs). Understanding the underlying GRN of this important cell population will provide insights for future directed reprogramming experiments aimed at potential therapies for congenital heart defects.
Learn more about the AHA Career Development Award here.
MCB Welcomes New Faculty James Nuñez
We are thrilled to welcome (back) James Nuñez to MCB! This July, Nuñez will join the MCB Biochemistry, Biophysics and Structural Biology division as an Assistant Professor.
Nuñez is a Doudna lab alum and is currently a postdoc in Jonathan Weissman's lab at UCSF, where he developed CRISPR-based technologies for editing the human epigenome.
These tools enable controlled changes in transcriptional states that are inherited through many cell divisions and cellular differentiation without altering the DNA sequence, thereby programming an 'epigenetic memory' of gene expression.
His research group will combine genome-wide CRISPR screens, cell biology, and biochemistry to understand how epigenetic memory and inheritance are established and maintained in mammalian cells, as well as engineer new technologies for perturbing the human epigenome and rewiring gene expression programs.
To learn more, follow Dr. Nuñez on Twitter @jamesknunez
MCB Welcomes New Faculty Megan Martik
We are thrilled to announce Megan Martik will be joining the MCB Genetics, Genomics and Development division as an Assistant Professor, arriving July 2021!
Martik is currently a postdoc in Dr. Marianne Bronner’s lab at the California Institute of Technology, where she has unraveled a gene regulatory network controlling the development of neural crest-derived cardiomyocytes and has found that these cells are required for adult heart repair in zebrafish. She’s also uncovered a gene circuit that has evolved progressively throughout vertebrate evolution to give rise to novel neural crest-derived cell types. Her research group will combine state of the art developmental biology techniques with systems-level approaches, such as single-cell transcriptomics and epigenomic analysis, to characterize gene networks controlling neural crest differentiation during development and neural crest contributions to adult regeneration in a variety of model organisms.
To learn more, follow Dr. Martik on Twitter @MeganMartikPhD
MCB Welcomes New Faculty Ian Swinburne
We are pleased to announce that Ian Swinburne is joining the MCB Cell & Developmental Biology division as an Assistant Professor, arriving in January 2021.
Swinburne is currently a postdoc at Harvard in Sean Megason’s lab where, within the inner ear, he found and characterized a previously unknown pressure-sensitive valve made of specialized cell-cell junctions. His research applies live-imaging, genetics, physiology, and bioinformatics to study zebrafish development with the goal of uncovering the mechanisms that control organ formation and function.
To learn more, follow Dr. Swinburne on Twitter @IanSwinburne